Poster | 6th Internet World Congress for Biomedical Sciences |
Margot M.S. Naaijkens(1), A.A. van Sorge(2)
(1)Rijnstate Hospital - ARNHEM. Netherlands
(2)Rijnstate Hospital Arnhem - ARNHEM. Netherlands
|
|
|
|
|
|
[Pharmacology] |
[Dermatology] |
PREPARATION
Composition Ibuprofen 5% 100 gram
Ibuprofen 5 gram N-Methylglucamine 4.73 gram Hydroxyethylcellulose 2 gram / 1.8 gram Kathon CG® 50 mg HCl Purified water ad 100 gram
Composition flurbiprofen 5% 100 gram
Flurbiprofen 5 gram N-Methylglucamine 4 gram Hydroxyethylcellulose 2 gram / 1.8 gram Kathon CG® 50 mg HCl Purified water ad 100 gram
PREPARATION PROTOCOL
Weigh all components.
Dissolve meglumine (N-Methylglucamine) in approximately 80 grams of water.
Subsequently, dissolve the ibuprofen/ flurbiprofen in the solution with meglumine (warming).
Correct the pH to 6.8 with HCl (solution has to stay transparent).
Weigh Kathon CG® (safety protocol).
Add Kathon CG® to the solution.
Put the solution in a tarred mortar.
Add hydroxyethylcellulose while mixing firmly.
Mix thorough until a smooth hydrogel is formed.
Add purified water up to a 100 grams.
Explanatory note to the usage of meglumine (N-Methylglucamine) and Kathon CG®
pH-series
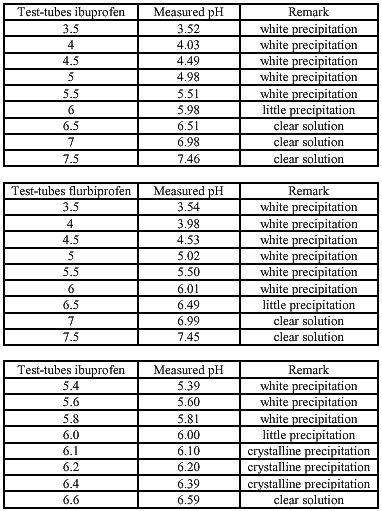
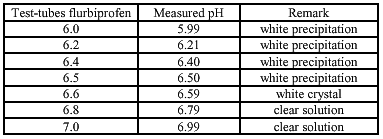
The solubility of ibuprofen/ flurbiprofen with equimolar quantities of meglumine is determent for different pHs. This is done to examine at which pH the components are dissolved (the lower pH the better for the conserving activity of Kathon CG®). The used pH-buffersolutions are made according to the study of Herzfeldt and Kümmel (1983) to the dissociation constants and solubility’s of NSAIDs.
Buffer solutions (Herzfeldt and Kümmel) (6)
BUFFER A
HCL 1M 14.1 ml
Glycine 75 mg
NaCl 552 mg
Aqua ad 150 ml
BUFFER B
Na2HPO4 . 2H2O 3.075 g
KH2PO4 420 mg
NaCl 22.5 mg
Aqua ad 150 ml
The different pHs are made by using different combinations of buffer A and B (pH-curve buffer solutions).
SOLUBILITY
Table 3
| Flurbiprofen | Flurbiprofensodium | |
| pH 6.8 | 10 mg in 6 ml (0.17%) | 10 mg in 2 ml (0.5%) |
| pH 7.2 | 10 mg in 3 ml (0.33%) | 10 mg in 2 ml (0.5%) |
1 Equimolar quantity (example):
M(NaOH)/M(ibuprofen)* x grams ibuprofen = x grams NaOH
LEGENDA MOLECULAR WEIGHT
| Compound | Molecular weight |
| Ibuprofen | 206,28 |
| Flurbiprofen | 244,3 |
| NaOH | 40,00 |
| Meglumine | 195,22 |
| Trometamol | 121,1 |
|
|
|
|
|
|
[Pharmacology] |
[Dermatology] |