Poster | 6th Internet World Congress for Biomedical Sciences |
Yasuji Matsuoka(1), Mitsuhiro Okazaki(2), Yuko Sekino(3), Yoshihisa Kitamura(4)
(1)Nathan Kline Inst. - Orangeburg. United States
(2)(4)Dept Neurobiol. Kyoto Pharm Univ - Yamashina. Japan
(3)Department of Neurobiology and behavior. Gunma University School of Medicine - Maebashi. Japan
|
|
|
|
|
|
[Cell Biology & Cytology] |
[Neuroscience] |
Effect of A1 adenosine receptor antagonist for:
--Neuronal Cells.
--Glial Cells.
--Neuronal Apoptosis.
Effect of A1 adenosine receptor ANTAGONIST against KA-induced neurodegeneration
MAP-2-immunoreactivity was widely observed in vehicle-injected rat hippocampus (Fig. 1A). CPT itself appeared not to have any effect on MAP-2-immunoreactivity (Fig. 1B). After the i.c.v. injection of KA, MAP-2-immunoreactivity was lost specifically in the CA3 (Fig. 1C). Pretreatment with CPT (10 mg/kg, i.p.), an A1 adenosine receptor antagonist, at 60 min before the injection of KA, resulted in a marked reduction in MAP-2-immunoreactivity in both CA1 and CA3 (Fig. 1D). Quantitatively, the MAP-2-immunoreactive area was significantly decreased only in the CA3 after the injection of KA alone (Fig. 2B). It did not change in the CA1 (Fig. 2A). In contrast, the combination of KA and CPT (KA/CPT), induced a significant loss of MAP-2-immunoreactive area in the CA1 (Fig. 2).
Effect of A1 adenosine receptor agonist AGONIST KA-induced neuronal cell loss
The coadministration of CHA, an A1 adenosine receptor agonist, with KA attenuated the loss of MAP-2-immunoreactivity in the CA3 induced by KA (Fig. 1E). In addition, the coadministration of CHA with KA/CPT also attenuated the neuronal cell loss in both the CA1 and the CA3 (Fig. 1F). Quantitatively, the loss of the MAP-2-immunoreactive area in the CA3 with the injection of KA alone was significantly attenuated by the administration of CHA (Fig. 3B). The administration of CHA significantly attenuated the loss of the MAP-2-immunoreactive area in both the CA1(Fig. 3A) and the CA3 (Fig. 3B) caused by the injection of KA/CPT.
**Jump to the Top of Page, Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7
Fig. 1: Effects of an A1 adenosine receptor antagonist (CPT) and agonist (CHA) on KA-induced neurodegeneration.
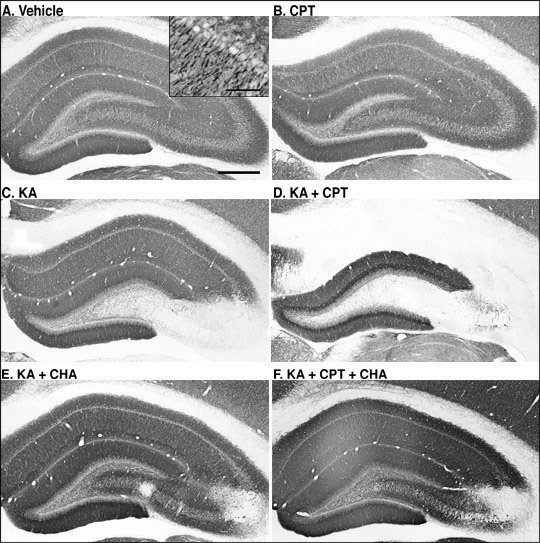
MAP-2-immunostained sections in the hippocampus 4 days after the injection.
**Jump to the Top of Page, Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7
Fig. 2: Quantitative analysis of KA-induced neuronal cell loss and the effect of an A1 adenosine receptor antagonist (CPT)
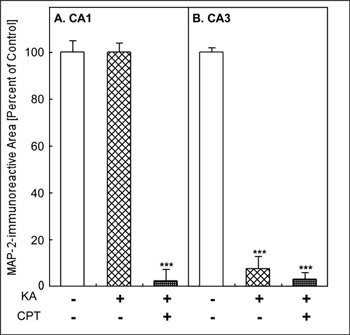
**Jump to the Top of Page, Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7
Fig. 3: Quantitative analysis of KA- or KA/CPT-induced neuronal cell loss and the effect of an A1 adenosine receptor agonist (CHA).
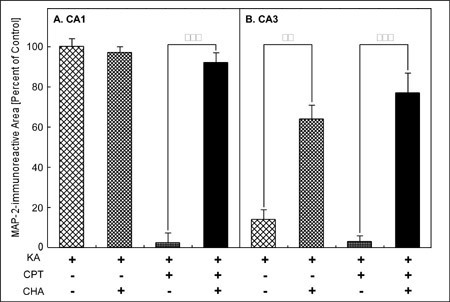
**Jump to the Top of Page, Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7
Histological changes of after coadministration of KA, CPT, and/or CHA
Injection of KA alone induced loss of the pyramidal neurons in CA3 (Fig. 4Bb), but not CA1 (Fig. 4Ba), although vehicle appeared not to have any effect on neurons (Fig. 4A). Pretreatment of CPT at 60 min before the injection of KA induced marked loss of neurons in both CA1 and CA3 (Fig. 4C). The coadministration of CHA with KA/CPT attenuated the neuronal cell loss in both the CA1 and the CA3 (Fig. 4D). Therefore, the histological changes, which examined using H-E staining, were conformity well with changes of MAP-2-immunoreactivity.
Fig. 4: Histological changes (hematoxylin and eosin staining)of rat hippocampal formation 4 days after injection of KA and effects of an A1 adenosine receptor antagonist (CPT) and agonist (CHA)
**Jump to the Top of Page, Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7
Changes in glial cells after KA-injection and the effect of adenosine receptor antagonist
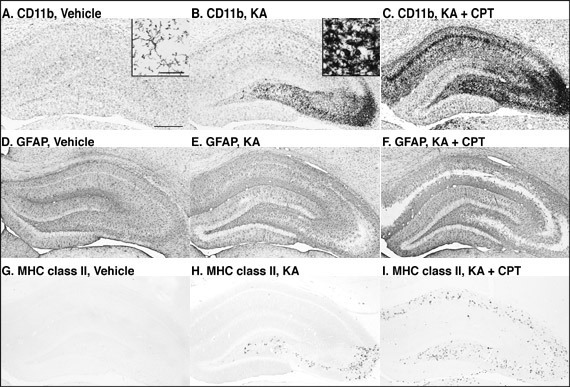
Microglia (CD11b-immunostaining) have thin and longer processes that are typically ramified (Fig. 4A). Four days after the i.c.v. injection of KA, numerous microglia with thick and shorter processes, i.e., typical ameboid type, were observed in the CA3 (Fig. 4B inset), while the microglia in the other hippocampal subfields were mostly ramified (Fig. 4B). After treatment with KA/CPT, ameboid microglia with thick and shorter processes were also observed in the CA1 (Fig. 4C). Astrocytes (GFAP-immunostaining) were slightly activated by injection with KA (Figs. 4D-4F). MHC class II-immunoreactivity was undetectable in the vehicle-injected rat hippocampus (Fig. 4G). Four days after KA-injection, MHC class II-immunoreactivity was observed only in the CA3 (Fig. 4H). Treatment with KA/CPT also induced MHC class II-immunoreactivity in the CA1 (Fig. 4I). The regions in which glial activation occurred correlated well with those that showed neurodegeneration, as judged by MAP-2-immunoreactivity.
Fig. 5: Photomicrographs of CD11b-, GFAP-, and MHC class II-immunostained sections in the hippocampus 4 days after the injection.
**Jump to the Top of Page, Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7
Phosphorylation of c-Jun after KA-injection and the effects of an adenosine receptor antagonist and agonist
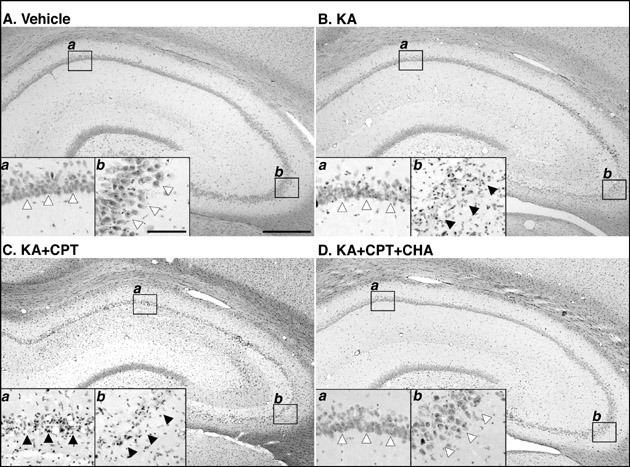
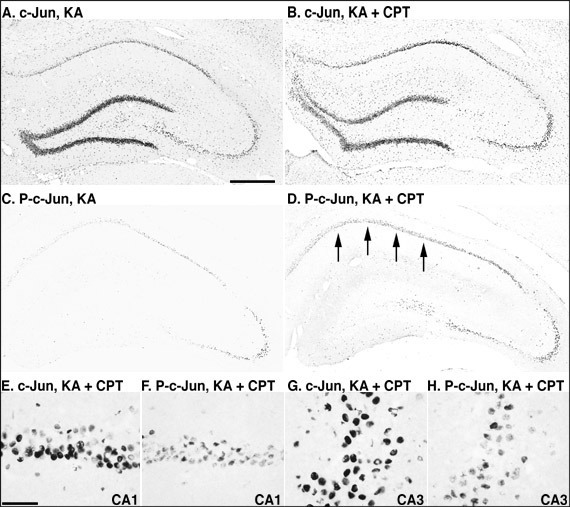
In the vehicle-injected rat hippocampus, c-Jun was detected in the dentate gyrus, while phosphorylated c-Jun was not detected in the rat hippocampus. Twelve hours after the injection of KA or KA/CPT, c-Jun was markedly induced throughout the hippocampus (Figs. 5A and 5B). In contrast, c-Jun phosphorylation was detected dominantly in CA3 pyramidal neurons, and slightly in CA1 (Fig. 5C). When CPT was injected 60 min before KA injection, c-Jun phosphorylation was markedly induced in the CA1 (Fig. 5D). c-Jun phosphorylation was observed in some c-Jun-immunoreactive pyramidal neurons in both the CA1 (Figs. 5E and 5F) and the CA3 (Figs. 5G and 5H).
Quantitatively, treatment with KA alone or KA/CPT significantly increased the number of c-Jun immunoreactive cells in the CA1 and the CA3 (Fig. 6A). Although phosphorylated c-Jun was not detected in the CA1 or the CA3, phosphorylated c-Jun significantly increased after the injection of KA alone or KA/CPT in both the CA1 and CA3 (Fig. 6B). The phosphorylation of c-Jun in the CA3 was greater than that in the CA1 after the injection of KA alone. c-Jun phosphorylation was significantly enhanced in the CA1 by the injection of KA/CPT in comparison to that with KA alone.
Fig. 6: Photomicrographs of c-Jun and phosphorylated-c-Jun- (P-c-Jun) immunostained sections in the hippocampus 12 hours after the injection of KA or the combination of KA and CPT.
**Jump to the Top of Page, Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7
Fig. 7: Quantitative analysis of the number of c-Jun- and phosphorylated-c-Jun-immunoreactive cells 12 hours after the injection of KA with or without CPT.
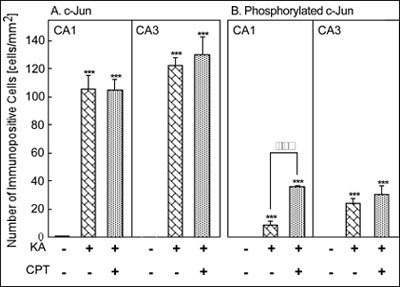
***P<0.001 versus vehicle injection, and õõP<0.01 versus injection of KA alone using ANOVA.
**Jump to the Top of Page, Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7
|
|
|
|
|
|
[Cell Biology & Cytology] |
[Neuroscience] |