Poster | 6th Internet World Congress for Biomedical Sciences |
Pilar Gutiérrez Navarro(1), Iluminada Hernández(2), Pedro Martínez de las Parras(3), Abdelkrim El Bekkouri(4), Raquel García(5)
(1)Facultad de Farmacia. Universidad de Granada. - Granada. Spain
(2)Departamento de Química Física.. Universidad de Granada - Granada. Spain
(3)Dpto. Química Física. Universidad de Granada - Granada. Spain
(4)(5)Dpto. Quimica Fisica. Universidad de Granada - Granada. Spain
|
|
|
[Biophysics] |
|
|
[Biochemistry] |
Bacterial resistence to the ß - lactam antibiotics is atributted to the presence of a family of enzymes, the ß -lactamases, that render the antibiotic inactive by hydrolyzing the C-N bond. The enzimes have been grouped into four classes(1,2) Classes A, C and D use an active site serine as a nucleophile. Class B ß - lactamases employ metal ions ( Zn [II], Cd [II], Co[II] or Mn[II] ) (3,4) ; Zn [II] or Cd [II] (5) ; one or two zinc ions (6) to effect ß - lactam cleavage. There are no clinically useful inhibitors of these metallo-enzymes , although the metal chelators EDTA and 1,10-o-phenantroline, and some mercurial chelators have shown inhibitory activity in vitro(7). Recently, a series of mercaptoacetic acid thiol esters and the saicyloyl cyclic phosphate have been shown to inhibit some of the metalloenzymes (8,9) . Therefore, at the moment, there is not specific inhibitors useful in therapeutical treatment because of the mechanism of action of the enzymes remains still unclear. The estudies currently devoted to the metalloenzymes are focused on the interaction between the enzyme and the metal ion in order to establish the role of the different metal ions in the catalytic activity (10,11).
The class B ß - lactamases have initilly received little attention because this class of enzymes was produced by only relatively innocuous Bacillus cereus strains. More recently, identification of some zinc ß -lactamase producing pathogenic strains of Bacteroides fragilis, Aeromonas, Serratia, Stenotrophomonas has increased the interest for this class of enzymes (10). The fact that metalloenzymes hydrolyse almost all ß - lactam antibiotics , including carbapenems and that they are not sensitive to dthe classical ß - lactamase inhibitors has increased enormously the interest for this class of enzymes (10).
Taking into account the interest of the interaction of the metal ion with the antibiotic, we have carried out a kinetic study of the degradation of a ß - lactam antibiotic , ampicillin, in the presence of those metal ions which are related to the metalloenzymes. Absorption and fluorescence data have been used to monitor the antibiotic decomposition reaction allowing us to obtain the kinetic equation.
Substances and reagents
Sodium ampicillin (99%) was purchased from Aldrich.. Anhydrous cadmium chloride, cobalt nitrate hexahidrate and manganese clroride tetrahidrate were obtained from Merck, Anhydrous zinc chloride from Sigma and methanol of over 99% purity was purchased from Merck.
Instrumentation
A Shimadzu ( Kyoto, Japan ) RF 5000 spectrofluorimeter equipped with a Julabo F 10 thermostat was used for fluorescence measurements . We also used a Perkin- Elmer Lambda 16 spectrofhotometer equipped with a Selecta thermostat ( 0.1 ºC) and a computer equipped with UV Winlab software from Perkin- Elmer. All measurements were performed with 1cm thick spectrophotometric cells.
Kinetic measurements
The kinetic study was carried out from intensity of fluorescence and absorption measurements. All kinetic experiments were performed at 25º C in methanol.
The kinetic mixtures were prepared directly on the spectrophotometric cell. In all kinetic experiences, solutions of appropriate concentration were prepared to obtain the desired concentration on the cell. The most diluted reagent was pipetted and placed on the cell inside the spectophotometer. Once termal equilibrium was achieved, a 100 l volume of a solution of appropiate concentration of the other reagent was added.
The kinetic reaction of ampicillin catalysed by Cd2+ was studied in a n earlier work(12) and was followed by the appearance of chromophore near 280 nm. The appearance of this absorption band was attributed to the breaking of the ß -lactam ring and the formation of the corresponding penamaldic derivative.
The degradation reaction in presense of Zn2+ have also followed by the appearance of the band at 282 nm, and the Co2+ and Mn2+ reactions changes absorption at 331 and 250 , respectively. In the kinetic study a series of methanolic solutions were assayed containing different excesses of ampicillin ( ranging from 1 to 12 x 10-4 M ) and constant metal concentration ( 1x 10-4 M ); and also in ampicillin solutions of constant concentration ( 1x10-4 M ) and different metal ion concentration ( ranging from 0 to 750 x 10-4 M). Initial rates ( Vobs ) of product appearance , in this case, were calculated in the same way as reported earlier12 for the reactión in the presence of Cd2+.
In the study corresconding to Co2+ and Mn2+ , the initial rate constants of ampicillin degradation were obtained by measuring the change the absorbance, at appopiate wavelengh, with respect to the total change.
The degradation reaction catalysed by Zn2+ were also monitored fluorimetrically at the relevant excitation and emision wavelengths. In the first times, one hour, the formation of a fluorescent product is observed for the reaction catalalysed by Zn2+ , whereas for the reactions catlatysed by other metal ions considered the appearance of fluorescence emission is also observed after 24 hours of reaction.
Excitation and emision spectra
In all experiments the fluorescence measurements were made at 25 º C .The uncorrected excitation and emission spectra corresponding to each of the systems considered were obtained from kinetic mixtures with each of the metal ions considered.
The UV spectrum changes that occur in degradation of ampicillin catalysed by Zn2+ are shown in fig. 1a. The increase of absoption near 280 nm has been assigned to the ligand- to- metal tranfer band of the complex of the penamaldic derivative of ampicillin (12,14). Fig 1b shows the formation of the fluorescent product thar occur simultaneously with the UV spectrum changes. The uncorrected excitation and emission spetra of the fluoescent reaction product which is formed , are shown in Fig 2.
Figure 3 shows the initial rate values for different excess of metal ion ( Co2+, Mn2+ and Zn2+) and constant ampicillin concentration. It can be seen that there is and increasing rate when the concentration of Zn2+ increases (Fig. 3c), and that avobe a certain concentration there is a tendency to a constant value. We can thus deduce that catalysed degradation occurs with ampicillin- Zn2+ intermediate complexes. Also, Fig 3c is indicative that the 1:1 complex , ampicillin-Zn2+ , is degraded with an appreciable rate. In experiments carried out in excess of ampicillin , the initial rate values decrease when the ampicillin concentration is increased (Fig 4 ) . This fact can explain for the formation of two complexes ampicillin - Zn2+, which are decomposed at different rate values.
The previous discussion togheter the profiles absorbance- time for ampicillin-zinc system suggest that the complete scheme of the kinetic reaction is:
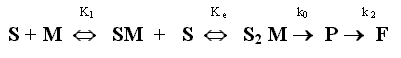
where S represents intact ampicillin, M metal ion, SM and S2 M are two complexes formed between intact ampicillin and Zn2+ ion of stoichiometries 1:1 and 2:1, respectively. K1 is the formation constant of SM compound, Ke that of S2 M from SM. The complex SM is also decomposed for to give P in a first order reaction, where the first order rate constant is k, which has not been included in the scheme for simplicity. The value of k0, correspomding to a zero order process can be considered negligible with respect to k1 value.
The k1 and k2 values have been calculated from fluoescence intensity data obtained of a kinetic experiment with a large excess of Zn2+ ( 750:1, S2M concentration negligible). The fluorescence data at 450 nm were fitted to a consecutive reactions using the Newton Raphson least-squares method. The k1 and k2 values are shoun in Table I.
Co2+ reacts with ampicillin giving rise to three complex species of 1:1, 2:1 and 3:1 stoichiometries recpectively13 . This fact togheter the results shwn in Fig. 2a suggest that the complex 1:1 has very big kinetic stability. The 2:1 complex has also comsiderable thermal stabillity, as can be seen in fig 2a, where the initial rate values for a excess of antibiotic are plot versus ampicillin concentration. Therefore, the first order constant value (k1) for the intermediate 1:1, ampicillin- cobalt can be considered negligible..
In fig 2a we can be see that ampicillin with the Mn 2+ form complexes wchich have a grand kinetic stability and that the 1:1 specie has the least stabitity. The rate constant for this complex is also shows in Table I.
In terms of ampicillin- metal ion -methanol system as a potential model for the zinc-dependent ß -lactamases, it is interesting to note that other metal ions may replace zinc in the in the enzyme with some retention of activity 15. It has been reported that replacement with Co2+ ion gives an enzyme with 12.6% of the activity of the zinc-containing enzyme. With Mn2+ and Cd2+ the activity was reduced to 6.7 and 1%, respectively. No activity was noted with Cu2+ and Ni2+ . Thus, in ours case, the activity depends of metal ion, but there is not total similarity between the relative order of activity of metal ions in the enzime and this model system.
|
|
|
[Biophysics] |
|
|
[Biochemistry] |