Poster | 6th Internet World Congress for Biomedical Sciences |
Pilar Gutiérrez Navarro(1), Iluminada Hernández(2), Pedro Martínez de las Parras(3), Abdelkrim El Bekkouri(4), Raquel García(5)
(1)Facultad de Farmacia. Universidad de Granada. - Granada. Spain
(2)Departamento de Química Física.. Universidad de Granada - Granada. Spain
(3)Dpto. Química Física. Universidad de Granada - Granada. Spain
(4)(5)Dpto. Quimica Fisica. Universidad de Granada - Granada. Spain
|
|
|
[Biophysics] |
|
|
[Biochemistry] |
The UV spectrum changes that occur in degradation of ampicillin catalysed by Zn2+ are shown in fig. 1a. The increase of absoption near 280 nm has been assigned to the ligand- to- metal tranfer band of the complex of the penamaldic derivative of ampicillin (12,14). Fig 1b shows the formation of the fluorescent product thar occur simultaneously with the UV spectrum changes. The uncorrected excitation and emission spetra of the fluoescent reaction product which is formed , are shown in Fig 2.
Figure 3 shows the initial rate values for different excess of metal ion ( Co2+, Mn2+ and Zn2+) and constant ampicillin concentration. It can be seen that there is and increasing rate when the concentration of Zn2+ increases (Fig. 3c), and that avobe a certain concentration there is a tendency to a constant value. We can thus deduce that catalysed degradation occurs with ampicillin- Zn2+ intermediate complexes. Also, Fig 3c is indicative that the 1:1 complex , ampicillin-Zn2+ , is degraded with an appreciable rate. In experiments carried out in excess of ampicillin , the initial rate values decrease when the ampicillin concentration is increased (Fig 4 ) . This fact can explain for the formation of two complexes ampicillin - Zn2+, which are decomposed at different rate values.
The previous discussion togheter the profiles absorbance- time for ampicillin-zinc system suggest that the complete scheme of the kinetic reaction is:
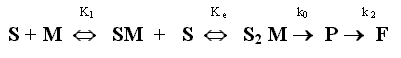
where S represents intact ampicillin, M metal ion, SM and S2 M are two complexes formed between intact ampicillin and Zn2+ ion of stoichiometries 1:1 and 2:1, respectively. K1 is the formation constant of SM compound, Ke that of S2 M from SM. The complex SM is also decomposed for to give P in a first order reaction, where the first order rate constant is k, which has not been included in the scheme for simplicity. The value of k0, correspomding to a zero order process can be considered negligible with respect to k1 value.
The k1 and k2 values have been calculated from fluoescence intensity data obtained of a kinetic experiment with a large excess of Zn2+ ( 750:1, S2M concentration negligible). The fluorescence data at 450 nm were fitted to a consecutive reactions using the Newton Raphson least-squares method. The k1 and k2 values are shoun in Table I.
Co2+ reacts with ampicillin giving rise to three complex species of 1:1, 2:1 and 3:1 stoichiometries recpectively13 . This fact togheter the results shwn in Fig. 2a suggest that the complex 1:1 has very big kinetic stability. The 2:1 complex has also comsiderable thermal stabillity, as can be seen in fig 2a, where the initial rate values for a excess of antibiotic are plot versus ampicillin concentration. Therefore, the first order constant value (k1) for the intermediate 1:1, ampicillin- cobalt can be considered negligible..
In fig 2a we can be see that ampicillin with the Mn 2+ form complexes wchich have a grand kinetic stability and that the 1:1 specie has the least stabitity. The rate constant for this complex is also shows in Table I.
In terms of ampicillin- metal ion -methanol system as a potential model for the zinc-dependent ß -lactamases, it is interesting to note that other metal ions may replace zinc in the in the enzyme with some retention of activity 15. It has been reported that replacement with Co2+ ion gives an enzyme with 12.6% of the activity of the zinc-containing enzyme. With Mn2+ and Cd2+ the activity was reduced to 6.7 and 1%, respectively. No activity was noted with Cu2+ and Ni2+ . Thus, in ours case, the activity depends of metal ion, but there is not total similarity between the relative order of activity of metal ions in the enzime and this model system.
|
|
|
[Biophysics] |
|
|
[Biochemistry] |