Poster | 6th Internet World Congress for Biomedical Sciences |
Margot M.S. Naaijkens(1), A.A. van Sorge(2)
(1)Rijnstate Hospital - ARNHEM. Netherlands
(2)Rijnstate Hospital Arnhem - ARNHEM. Netherlands
|
|
|
|
|
|
[Pharmacology] |
[Dermatology] |
The administration of drugs via the skin has benefits above oral administration. It is a non-invasive administration and is suitable for people who cannot use drugs orally (unconscious or vomiting). Also, the first-pass metabolism and gastro intestinal side effects can be avoided. However, it is difficult to reach therapeutic levels because of the difficulty for the druf to penetrate the skin barrier. Another disadvantage is inter-individual differences in penetration of the skin. Also, intra-individual differences do appear, because of the existence of multiple skin-types on one individual. To administrate drugs through the skin, the drug itself must comply to certain factors. The drug has to have, among others, a strong potency, must not exceed a certain molecular size (MW under 500 Dalton) and must not be irritating to the skin. The penetration of drugs through the skin can be influenced by the compounds in the topical preparation (1,2).
This study looks at the possibilities of the development of a 5% flurbiprofen hydrogel. The formulation of several commercial ibuprofen hydrogels was used as a starting point. These ibuprofen hydrogels are used for the treatment of muscle pain, soft tissue rheumatism and sport injuries. The hydrogel formulation is chosen because several studies with ibuprofen show that the hydrogel reaches higher plasma concentrations in shorter time than for example cream or unguent. In a study by Seth (3) different topical formulations (hydrogel, hydrophilic ointment, emulsion cream) of ibuprofen are compared. The parameters used are Cmax (maximum blood concentration) and Tmax (the time required for appearance of Cmax). This study shows that hydrogel reaches the highest Cmax with the shortest Tmax. The hydrophilic ointment shows the longest Tmax and lowest Cmax, the results of the emulsion cream are in between. The explanation given is that the water/ethanol mixture in the hydrogel increases the penetration and the absorption of ibuprofen through the skin to a greater extent than the lipophilic phase of the ointment and the emulsion cream. A study of Treffel (4) also shows higher epidermal concentration by using ibuprofen hydrogels than ibuprofen emulsions. Dominikus (5) has compared oral administration (tablet) with topical administration (hydrogel) of ibuprofen. Concentrations of ibuprofen in tissue and blood were examined. It shows that oral administration resulted in higher concentrations of ibuprofen in e.g. blood plasma and synovial fluid while topical administration gives higher concentrations in muscle tissue and subcutis.
The objective of this study is to prepare a clear and transparent flurbiprofen hydrogel with good consistency and cosmetic appearance. Both 5% ibuprofen as well as 5% flurbiprofen hydrogels are made. Of both substances the differences in solubility and pH behaviour (solubility by different pHs) are examined.
PREPARATION
Composition Ibuprofen 5% 100 gram
Ibuprofen 5 gram N-Methylglucamine 4.73 gram Hydroxyethylcellulose 2 gram / 1.8 gram Kathon CG® 50 mg HCl Purified water ad 100 gram
Composition flurbiprofen 5% 100 gram
Flurbiprofen 5 gram N-Methylglucamine 4 gram Hydroxyethylcellulose 2 gram / 1.8 gram Kathon CG® 50 mg HCl Purified water ad 100 gram
PREPARATION PROTOCOL
Weigh all components.
Dissolve meglumine (N-Methylglucamine) in approximately 80 grams of water.
Subsequently, dissolve the ibuprofen/ flurbiprofen in the solution with meglumine (warming).
Correct the pH to 6.8 with HCl (solution has to stay transparent).
Weigh Kathon CG® (safety protocol).
Add Kathon CG® to the solution.
Put the solution in a tarred mortar.
Add hydroxyethylcellulose while mixing firmly.
Mix thorough until a smooth hydrogel is formed.
Add purified water up to a 100 grams.
Explanatory note to the usage of meglumine (N-Methylglucamine) and Kathon CG®
pH-series
The solubility of ibuprofen/ flurbiprofen with equimolar quantities of meglumine is determent for different pHs. This is done to examine at which pH the components are dissolved (the lower pH the better for the conserving activity of Kathon CG®). The used pH-buffersolutions are made according to the study of Herzfeldt and Kümmel (1983) to the dissociation constants and solubility’s of NSAIDs.
Buffer solutions (Herzfeldt and Kümmel) (6)
BUFFER A
HCL 1M 14.1 ml
Glycine 75 mg
NaCl 552 mg
Aqua ad 150 ml
BUFFER B
Na2HPO4 . 2H2O 3.075 g
KH2PO4 420 mg
NaCl 22.5 mg
Aqua ad 150 ml
The different pHs are made by using different combinations of buffer A and B (pH-curve buffer solutions).
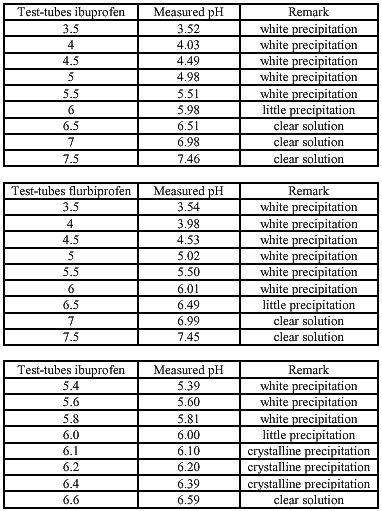
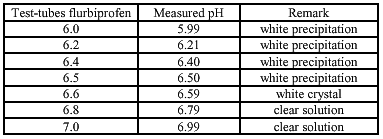
SOLUBILITY
Table 3
| Flurbiprofen | Flurbiprofensodium | |
| pH 6.8 | 10 mg in 6 ml (0.17%) | 10 mg in 2 ml (0.5%) |
| pH 7.2 | 10 mg in 3 ml (0.33%) | 10 mg in 2 ml (0.5%) |
1 Equimolar quantity (example):
M(NaOH)/M(ibuprofen)* x grams ibuprofen = x grams NaOH
LEGENDA MOLECULAR WEIGHT
| Compound | Molecular weight |
| Ibuprofen | 206,28 |
| Flurbiprofen | 244,3 |
| NaOH | 40,00 |
| Meglumine | 195,22 |
| Trometamol | 121,1 |
Taking the above mentioned results into account the preparation of Ibuprofen and Flurbiprofen gels is as follows:
Composition Ibuprofen hydrogel 5% 100 gram
Ibuprofen 5 gram Meglumine 4.73 gram Hydroxyethylcellulose 2 gram / 1.8 gram Kathon CG® 50 mg HCl Purified water ad 100 gram
Composition Flurbiprofen hydrogel 5% 100 gram
Flurbiprofen 5 gram Meglumine 4 gram Hydroxyethylcellulose 2 gram / 1.8 gram Kathon CG® 50 mg HCl Purified water ad 100 gram
Meglumine is essential to obtain a clear and transparent 5% flurbiprofen hydrogel. The optimal pH is established at 6.8. At this pH the flurbiprofen is still soluble and the conservative capacity of Kathon CG® is preserved. The consistency is still a problem. At 1.8% hydroxyethylcellulose it is median and the cosmetic appearance is good. At 2% the consistency is good but the cosmetic value becomes less. With dermal application of the clear, transparent 2%- gel it becomes cloudy and sticky, while using the 1.8%-gel this does not happen and the gel soaks into the skin.
Clotting during preparation of the 2% hydrogel by hand can be overcome by use of a rotor-statormixer.
A clear transparant 1.8 - 2% hydrogel with either 5% Ibuprofen or Flurbiprofen can be developed. The in vitro-release of flurbiprofen from the hydrogel as also the bioadhesive characteristics (7,8) will be subject of further research.
|
|
|
|
|
|
[Pharmacology] |
[Dermatology] |