

|
Andréa Rodrigues Cordovil Pires*, Felipe da Matta Andreiuolo**, Simone Rabello de Souza** |
|||||||||||||||||||||||||||||||||||||||||
|
Tissue microarrays are becoming a very useful tool for research and immunohistochemistry and in situ hibridization quality control methods. We developed a new technique that allows to build high density paraffin blocks without using a recipient block for the tissue cores and without using a commercial TMA builder instrument. This technique is based on the construction of TMA needles modifying conventional hypodermic needles and using a computer-generated grid to align the cores on the block mould, that is filled with liquid paraffin. We constructed more than 100 TMA blocks using this metthod, to use as immunohistochemistry and histochemistry positive and negative controls. This technique has the following advantages: is easy to reproduce, affordable to any Pathology laboratory, quick and creates uniform blocks, with up to over 100 cores aligned, adherent and easy to cut, with negligible losses during cutting and immunohistochemistry and in situ hibridization procedures. This work was published in Diagnostic Pathology, in 2006. Click here to review full text article.
|
||||||||||||||||||||||||||||||||||||||||||
|
|
Tissue microarrays (TMA) are becoming a very useful tool for research and quality control on immunohistochemistry (IHC) and in situ hibridization methods(1,2,3). The conventional construction of a TMA block involves the use of a custom needle or a commercial TMA builder instrument to punch the cores from donor blocks and the transference of these tissue cores to a recipient block, producing blocks with even 1000 tissue cores. This technique has some disadvantages:
This study was carried out in order to produce TMA blocks without the disadvantages listed above.
|
|||||||||||||||||||||||||||||||||||||||||
|
|
Needles (figure 1): Conventional hypodermic Becton-Dickinson PrecisionGlide® needles (table 1) were prepared for punching the donor blocks, as follows: we used a Dremel Multi-Pro® Rotary Tool Model 395, at low speed, with a cutting wheel atached, to cut off the bevel and straighten the tip. Using the same tool, lateral openings (windows) were made, 1,0mm away from the new tip, with around 8,0mm lenght and sligthly wider than half the needles' width, so that the punched core could be safely removed through it. Then the external surface of the needle was sharpened with the sanding surface of the cutting wheel. The internal surface was also sharpened using either a shaft cone stone or a metal sharpener. Core biopsies were manually punched out from the donor blocks, from regions selected on correspondent H&E slides overlayed. At this step it is important that the needle enters the block in a perpendicular fashion, so that the cores extracted will have a perfectly cylindrical shape. We alternatively adapted a hand-press grommet inserting machine, by means of removing the dies, and ataching one of the customized needles in their place, which ensures an even easier semi-automated movement, as well as a perpendicular angle between the needle and the donnor block. Grid (figure 2): We designed a grid using the drawing software Corel Draw® the following way: 1mm white circles were drawn and allingned leaving 1mm space between them, on a colorful background; the grid was printed in plain paper .
Blocks (figure 2): Once all cores were sticked to the mould, melted paraffin was gently poured on the mould. It is important to make sure that all cores are perfectly vertical at this step. From this point blocks were handled according to routine histopathological procedures. Sections, 4 mm thick, were cut in an American Optical standard rotatory microtome; each block provided 90-100 slides, depending on the tissue cores' length. There was no need to use adhesive-coated tape-sectioning. The design of each block was detailed in a TMA map, indicating positioning and identification of each core. We used normal tissues, different sized cores or position-specific blank cores for orientation during microscopy. Slides (figure 2b): Hematoxylin-eosin (H&E) stained slides were obtained and processed in the conventional way; those for immunohistochemistry were pre-treated with silane (3 amino-propyl-trietoxysilane) 3% in acethone. Immunohistochemistry was performed using standard manual procedures. Heat-mediated antigen retrieval was performed incubating slides in hot water bath at 96°C for 40 minutes.
Table 1: Needle measures and characteristics.
Table 2: Costs
|
|||||||||||||||||||||||||||||||||||||||||
|
|
Crafting the needles was easy and quick, requiring little skill to produce the bevel and lateral window - less than 10 minutes. It´s advisable to use protection eyeglasses and gloves in order to avoid harm from metal dust. The total initial cost was near USD$100.00 (table 2). The construction of TMA blocks using this technique was also quick (15 minutes to make one 15-core block ) and easy (once the H&E slide is marked, the technician can punch the cores and construct the block following the designed map). Initially, 79 different primary antibodies, monoclonal and policlonal, were tested succesfully for titering / protocol stablishment (75 from Biogenex® and 4 from Novocastra®) using 320 slides from TMA blocks containing 25 different samples from normal or neoplastic tissues each. Both 16 and 18 gauge needles yielded the same satisfactory results. After this antibody titering / protocol stablishment phase, smaller TMA blocks were made (6 to 9 tissue cores) to be used as routine IHC positive and negative controls. Each block was cut until one tissue core finished, resulting in 90-100 control slides. These control slides were stored in plastic slide boxes at 4°C for no longer than 4 weeks. Each IHC case slide had two areas: the control TMA just below slide identification and the case section just below the control TMA (figure 3). More than 100 TMA blocks have been confectioned up to the present. There were little core losses (<1%), most of them due to consumption of one or more tissue cores at microtomy, rarely to section falling off from slides during technical procedures. To avoid this irregular consumption, it is very important to produce cores with near the same tissue length, so one should avoid donnor blocks from which many slides have been cut or that bear too thin tissue specimens.
|
|||||||||||||||||||||||||||||||||||||||||
|
|
Recently there have been reports of alternative methods for the manual construction of tissue arrays(4-7). This article describes the alternative we developed and have been testing for near one year, which is inexpensive, easy and quick to perform, and produces high-density aligned TMA blocks that can be manipulated the same way any other paraffin tissue block. Following the present method, blocks do not break apart and there is minimum core losses, precluding the use of an adhesive tape sectioning device. The initial cost is near USD$100.00; aditional costs are negligible and include solely the eventual purchase of extra hipodermic needles for customization and adhesive tape. It is also possible to use bone marrow trephine biposy needles or other types of needles, however our customized needles have proven to be much more cost-effective, and the lateral opening is very useful. The use of the double face adhesive tape to stick cores was recently described(8), and we have developed a grid that allows for a satisfactory alignment of tissue fragments, and blocks thus obtained necessarily display tissue cores aligned and at the same cutting plane. Although the needles can be used mannualy, using the hand-press grommet inserting machine makes core punching easier, once is absolutely perpendicular, prevents damage to the donnor blocks and is faster.
|
|||||||||||||||||||||||||||||||||||||||||
|
|
We present with an alternative method for the contruction of high-density tissue array blocks that can be performed by small / low budget anatomic pathology laboratories, at low cost and requiring minimum skill and time.
|
|||||||||||||||||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||||||||||
|
|
- Marcial García Rojo (02/10/2005 19:43:14)
- Andréa Rodrigues Cordovil Pires (02/10/2005 23:57:02)
- José Javier López Caballero (05/10/2005 11:43:07)
- Clóvis Klock (07/10/2005 13:37:14)
- Cesáreo Corbacho Cuevas (25/10/2005 20:50:08)
- CLAUDIO ANDRÉS MIRABELLI (25/10/2005 20:52:15)
- Andréa Rodrigues Cordovil Pires (26/10/2005 0:45:17)
- Manuel Diaz-Marta Puentes (19/01/2012 20:58:45)
- Manuel Diaz-Marta Puentes (19/01/2012 20:58:46)
- Manuel Diaz-Marta Puentes (19/01/2012 20:58:46)
- Manuel Diaz-Marta Puentes (23/01/2012 20:16:17)
- Manuel Diaz-Marta Puentes (23/01/2012 20:16:17)
- Manuel Diaz-Marta Puentes (23/01/2012 20:16:17)
|
|||||||||||||||||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||||||||||||
Web mantenido y actualizado por el Servicio de informática uclm. Modificado: 16/06/2015 14:10:50
.jpg) fiogf49gjkf0dFigure 1a. Needle customization, step by step – cutting the tip (A to C), opening the lateral window (D to F) and creating a new bevel (G to I).">
fiogf49gjkf0dFigure 1a. Needle customization, step by step – cutting the tip (A to C), opening the lateral window (D to F) and creating a new bevel (G to I).">
 fiogf49gjkf0dFigure 1b. Paraffin block punctioning, manual (J and K) and with hand-press grommet inserting machine (L and M).">
fiogf49gjkf0dFigure 1b. Paraffin block punctioning, manual (J and K) and with hand-press grommet inserting machine (L and M).">
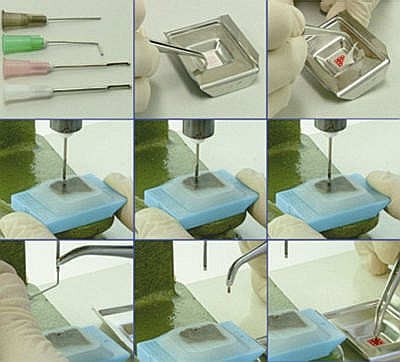 fiogf49gjkf0dFigure 2a. Custom-built needles (A), grid positionig with double-sided adhesive tape (B and C), block positioning on hand-press grommet inserting machine (D), punctioning tissue core (E and F), removing tissue core from the needle (G and H), positioning tissue core on the grid, over the double-sided adhesive tape (I).">
fiogf49gjkf0dFigure 2a. Custom-built needles (A), grid positionig with double-sided adhesive tape (B and C), block positioning on hand-press grommet inserting machine (D), punctioning tissue core (E and F), removing tissue core from the needle (G and H), positioning tissue core on the grid, over the double-sided adhesive tape (I).">
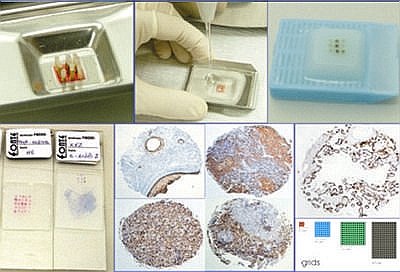 fiogf49gjkf0dFigure 2b. Grid complete with tissue cores in the mould (J), pouring liquid paraffin in the mould (K), TMA block with 9 tissue cores (L), slides with 25 and 9 tissue cores (M), immunohistochemistry stains done by 21G needle: cytokeratin AE1/AE3 - teratoma (N), LCA – thymus (O), CD20 – tonsil (P) and CD3 – tonsil (Q) and immunohistochemistry stain done by 16G needle: PLAP placenta; grids - 9, 49, 144 and 228 cores.">
fiogf49gjkf0dFigure 2b. Grid complete with tissue cores in the mould (J), pouring liquid paraffin in the mould (K), TMA block with 9 tissue cores (L), slides with 25 and 9 tissue cores (M), immunohistochemistry stains done by 21G needle: cytokeratin AE1/AE3 - teratoma (N), LCA – thymus (O), CD20 – tonsil (P) and CD3 – tonsil (Q) and immunohistochemistry stain done by 16G needle: PLAP placenta; grids - 9, 49, 144 and 228 cores.">